Write an Equation to Show How Hpo42ã¢ë†â€™ Can Act as an Acid With H2o Acting as a Base.
10.3: Water - Both an Acrid and a Base
- Page ID
- 16082
Learning Objectives
- To write chemical equations for water acting as an acrid and equally a base of operations.
Water (HiiO) is an interesting chemical compound in many respects. Hither, we will consider its ability to behave as an acid or a base.
In some circumstances, a h2o molecule volition accept a proton and thus act as a Brønsted-Lowry base. Nosotros saw an example in the dissolving of HCl in H2O:
\[\rm{HCl + H_2O_{(ℓ)} \rightarrow H_3O^+_{(aq)} + Cl^−_{(aq)}} \label{Eq1}\]
In other circumstances, a water molecule tin donate a proton and thus deed as a Brønsted-Lowry acid. For example, in the presence of the amide ion (see Example 4 in Section 10.2), a water molecule donates a proton, making ammonia as a product:
\[H_2O_{(ℓ)} + NH^−_{2(aq)} \rightarrow OH^−_{(aq)} + NH_{three(aq)} \label{Eq2}\]
In this instance, NH2 − is a Brønsted-Lowry base (the proton acceptor).
So, depending on the circumstances, H2O can human activity every bit either a Brønsted-Lowry acid or a Brønsted-Lowry base. H2o is not the only substance that tin can react as an acid in some cases or a base in others, but information technology is certainly the most mutual example—and the most of import one. A substance that can either donate or take a proton, depending on the circumstances, is called an amphiprotic compound.
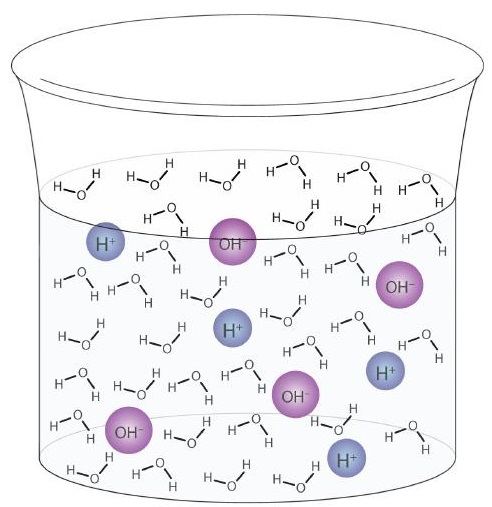
A h2o molecule can act as an acrid or a base fifty-fifty in a sample of pure h2o. About 6 in every 100 million (vi in xeight) h2o molecules undergo the following reaction:
\[H_2O_{(ℓ)} + H_2O_{(ℓ)} \rightarrow H_3O^+_{(aq)} + OH^−_{(aq)} \label{Eq3}\]
This process is called the autoionization of water (Figure \(\PageIndex{i}\)) and occurs in every sample of water, whether it is pure or function of a solution. Autoionization occurs to some extent in any amphiprotic liquid. (For comparison, liquid ammonia undergoes autoionization as well, merely only well-nigh 1 molecule in a 1000000 billion (one in 1015) reacts with another ammonia molecule.)
Example \(\PageIndex{ane}\)
Identify water as either a Brønsted-Lowry acrid or a Brønsted-Lowry base.
- H2O(ℓ) + NOtwo −(aq) → HNO2(aq) + OH−(aq)
- HC2HthreeO2(aq) + H2O(ℓ) → H3O+(aq) + CiiH3Otwo −(aq)
Solution
- In this reaction, the water molecule donates a proton to the NO2 − ion, making OH−(aq). Every bit the proton donor, H2O acts as a Brønsted-Lowry acid.
- In this reaction, the h2o molecule accepts a proton from HCtwoHiiiO2, becoming HthreeO+(aq). As the proton acceptor, HtwoO is a Brønsted-Lowry base.
Exercise \(\PageIndex{2}\)
Place h2o as either a Brønsted-Lowry acid or a Brønsted-Lowry base.
- HCOOH(aq) + HiiO(ℓ) → HthreeO+(aq) + HCOO−(aq)
- H2O(ℓ) + POfour three−(aq) → OH−(aq) + HPO4 2−(aq)
- Answer
-
ane. HtwoO acts equally the proton acceptor (Brønsted-Lowry base)
2. H2O acts as the proton donor (Brønsted-Lowry acrid)
Concept Review Exercises
- Explicate how water can deed as an acid.
- Explain how water tin human action as a base.
Answers
- Under the correct conditions, H2O can donate a proton, making information technology a Brønsted-Lowry acrid.
- Under the right conditions, HtwoO can accept a proton, making information technology a Brønsted-Lowry base.
Key Takeaway
- Water molecules can act as both an acid and a base of operations, depending on the atmospheric condition.
Exercises
- Is H2O(ℓ) acting as an acid or a base of operations?
HiiO(ℓ) + NH4 +(aq) → HiiiO+(aq) + NH3(aq)
- Is H2O(ℓ) acting equally an acid or a base?
CH3 −(aq) + H2O(ℓ) → CHiv(aq) + OH−(aq)
- In the aqueous solutions of some salts, one of the ions from the salt can react with water molecules. In some C2H3O2 − solutions, the post-obit reaction tin occur:
C2H3O2 −(aq) + H2O(ℓ) → HC2H3O2(aq) + OH−(aq)
Is HtwoO acting as an acrid or a base of operations in this reaction?
- In the aqueous solutions of some salts, one of the ions from the common salt tin can react with water molecules. In some NH4 + solutions, the following reaction can occur:
NHiv +(aq) + HiiO → NH3(aq) + H3O+(aq)
Is H2O acting equally an acid or a base in this reaction?
- Why is pure h2o considered neutral?
Answers
- base
- acid
- acrid
- base of operations
v. When h2o ionizes, equal amounts of H+ (acid) and OH− (base) are formed, and so the solution is neither acidic nor bones: HtwoO(ℓ) → H+(aq) + OH−(aq)
[SIDE Notation: It is rare to truly accept pure water. Water exposed to air will usually be slightly acidic because dissolved carbon dioxide gas, or carbonic acid, decreases the pH slightly below 7. Alternatively, dissolved minerals, like calcium carbonate (limestone), can make water slightly basic.]
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/The_Basics_of_GOB_Chemistry_(Ball_et_al.)/10%3A_Acids_and_Bases/10.03%3A_Water_-_Both_an_Acid_and_a_Base
0 Response to "Write an Equation to Show How Hpo42ã¢ë†â€™ Can Act as an Acid With H2o Acting as a Base."
Post a Comment